Philip Morris, submitted an application to the FDA for new tobacco vaporizer.
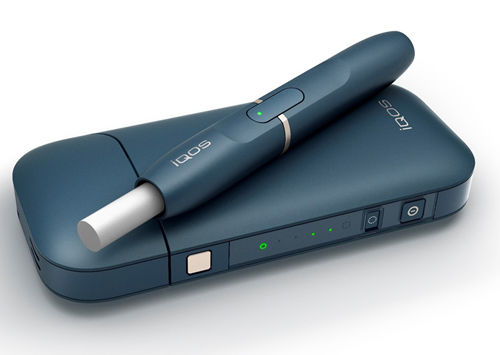
Philip Morris, maker of Marlboro cigarettes, submitted an application to the FDA on Tuesday seeking approval for its new tobacco vaporizer. The iQOS device, as it’s currently called, works on the same principle as the Pax, wherein the ground plant matter is gently heated until the active ingredients are vaporized, rather than burned with an open flame. Philip Morris claims that the vapor has 90 percent fewer harmful chemicals than normal cigarette smoke.
Rather than use a propylene glycol solution, the iQOS relies on replaceable real tobacco leaf cartridges which are shaped like conventional cigarettes. These “heat sticks” plug into the plastic heating element to produce a 500 degree F vapor and last around a dozen puffs.
If approved, the iQOS could profoundly shake up the US vaporizer market, which is currently dominated by modular, e-liquid-based vapes. The iQOS’ ease of use, low cost (heat stick packs cost around $6 on average), and brand familiarity are a potent marketing combination. The iQOS is already being sold in a number of foreign markets, including Japan where it has already captured nearly 2.4 percent of the market there.